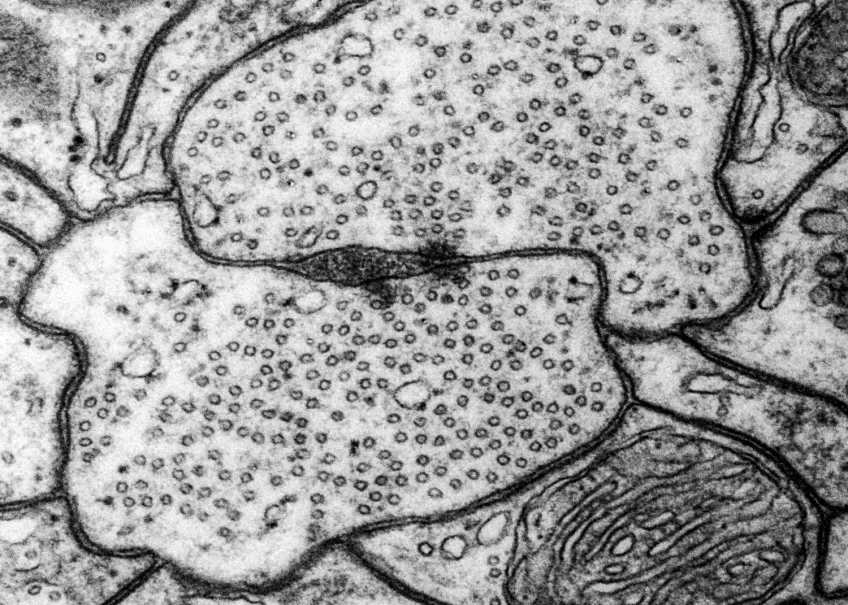
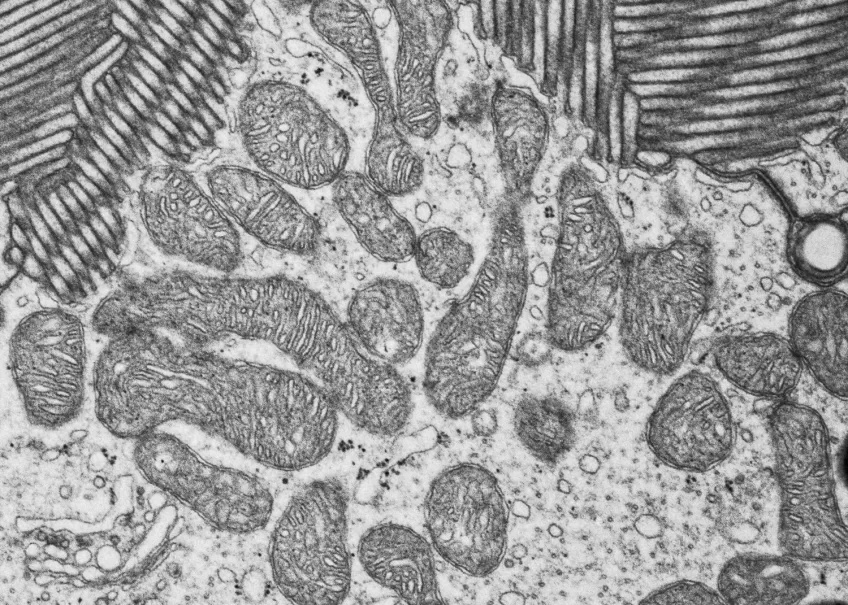
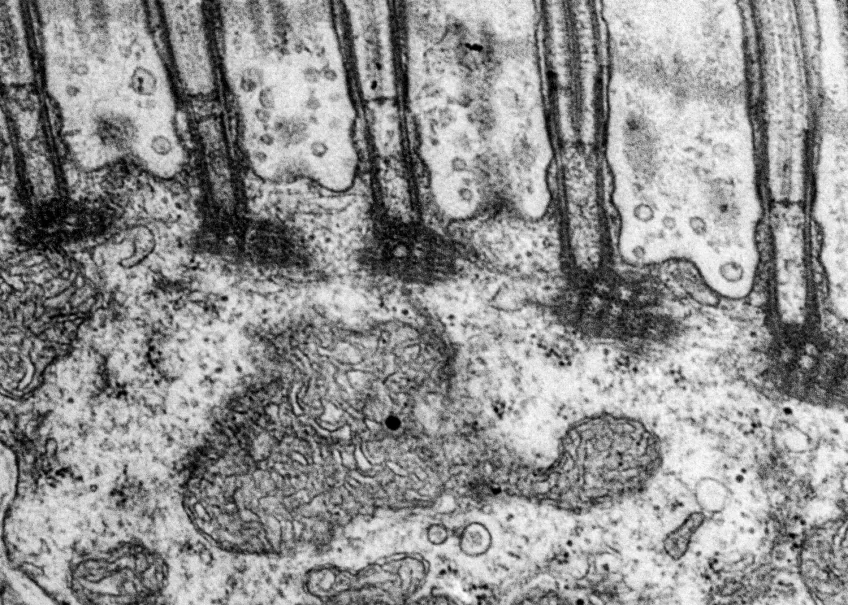
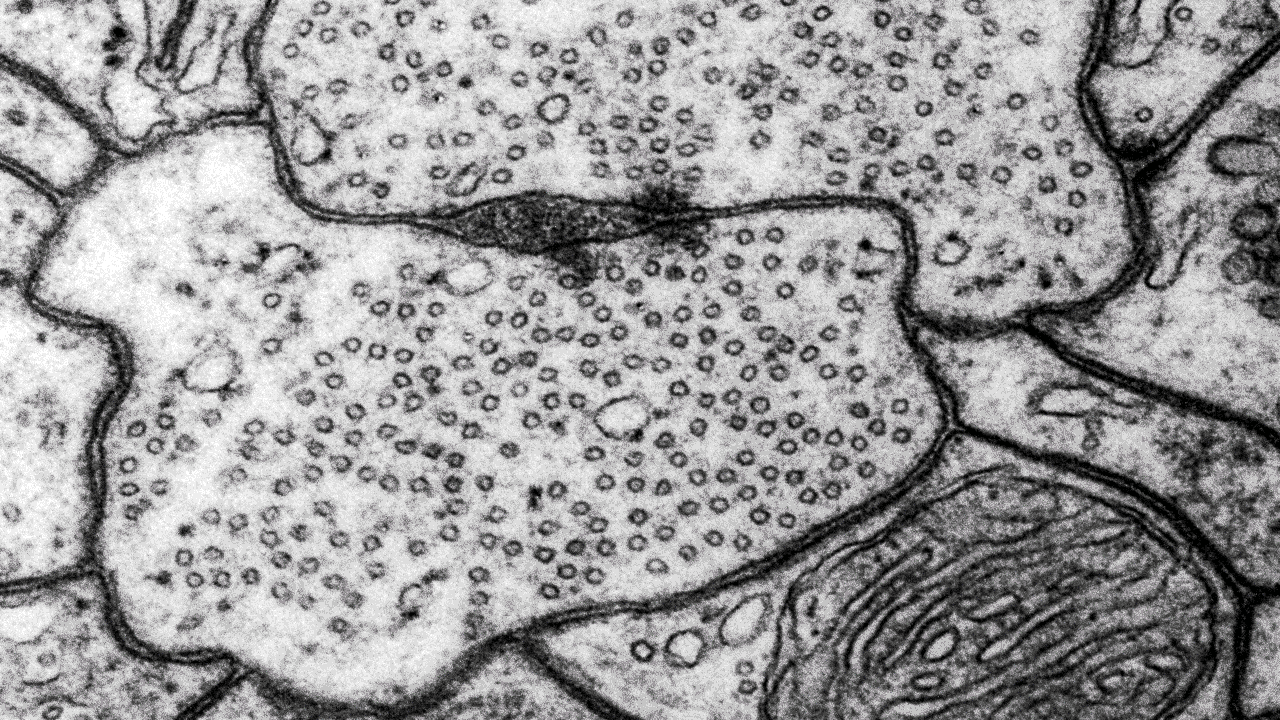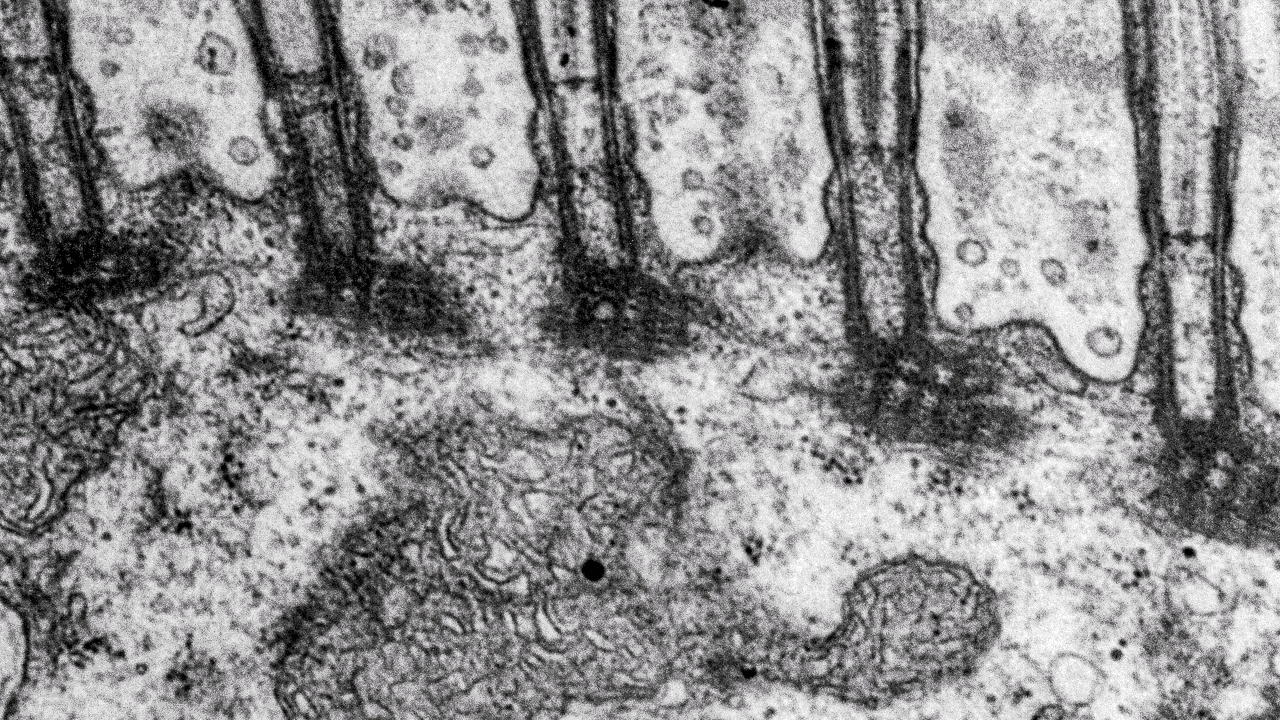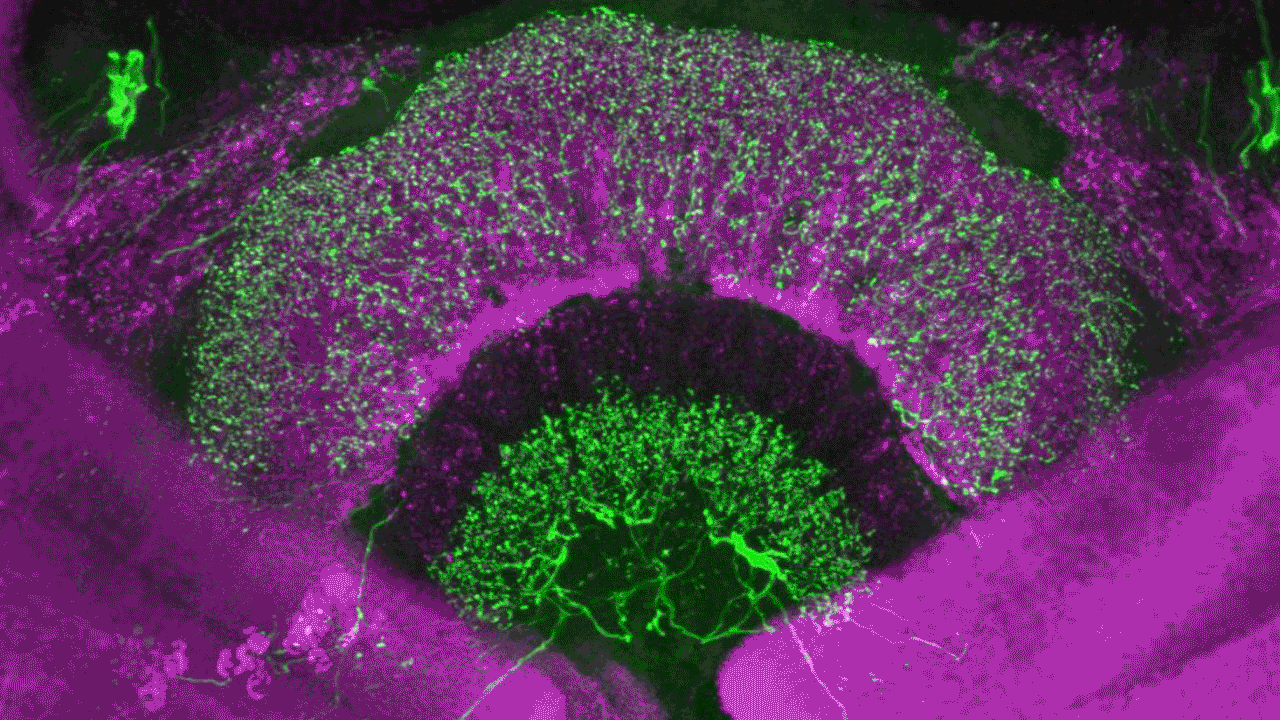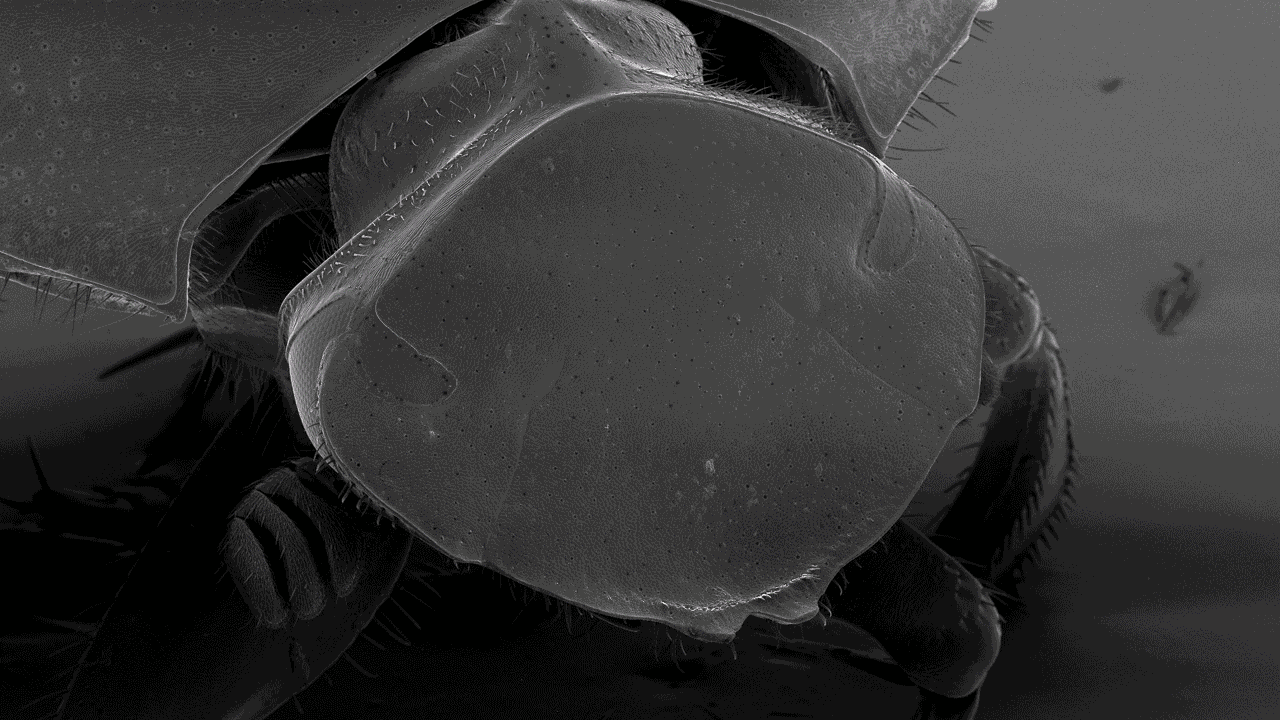
Microscopy platform
At our microscopy platform in Lund, you meet personnel with expertise and long experience working with biological material and microscopy, imaging, and analysis. Let us help you through the process or learn to do it yourself.
In our laboratories, you can use both standard microscope techniques, like scanning electron microscopy (SEM), transmission electron microscopy (TEM) and confocal laser scanning microscopy (CLSM), as well as infra-red based analysis techniques and micro-computed tomography (micro-CT).
We have the knowledge and microscopes to prepare your samples for further studies by synchrotron methods at MAX IV.
Besides working with human and mouse tissue, we also have experience with many other biological samples, such as animals, insects, plants, fossils, and even dinosaurs, the intestine of water fleas, and non-biological materials like stones, building materials, and computer chip.
We are open to researchers from within Lund University and to people from other universities and the industry.
Always contact us before you start sample preparation to make sure you don't waste precious material and time.
Experiment design and training
If you need help with sample preparation and experimental design we will help you. You can also learn how to use our equipment by yourself. After a training period, you get a "driver's license" and are allowed to book and use the instruments.
Courses in microscopy techniques
We offer the courses Common Microscopy Methods in Biological Research (external website) and Microscopy – Bioimaging (external website) for PhD and Master students.
User fees for using the microscopes, experimental design and training
We have different fees for the different kinds of microscopy. The fees depend on if you are an internal or external user. The Department of Biology contributes with 33% of the user fee for those utilising funds administered by the Department of Biology to cover this cost.
Confocal laser scanning microscopy Leica SP8
400 SEK per hour
Light sheet microscopy Leica DLS SP8
400 SEK per hour
Micro-computed tomography (Micro CT) Nikon XTH 225
600 SEK per hour
Scanning electron microscopy Hitachi SU3500
400 SEK per hour
Transmission electron microscopy JEOL 1400 Plus
600 SEK per hour
Staff support during imaging or sample preparation
600 SEK per hour
Sample preparation and lab support
Fixation and embedding in epoxy for TEM (max 10 samples)
1000 SEK
Ultrathin sectioning and counter staining for TEM
600 SEK per hour
Full sample preparation for SEM, including drying and coating (max 6 samples)
1000 SEK
SEM sample preparation including only mounting and sputter coating (max 6 samples)
600 SEK
Confocal laser scanning microscopy Leica SP8
400 SEK per hour
Light sheet microscopy Leica DLS SP8
400 SEK per hour
Micro-computed tomography (Micro CT) Nikon XTH 225
800 SEK per hour
Scanning electron microscopy Hitachi SU3500
700 SEK per hour
Transmission electron microscopy JEOL 1400 Plus
800 SEK per hour
Staff support during imaging or sample preparation
750 SEK per hour
Sample preparation and lab support
Fixation and embedding in epoxy for TEM (max 10 samples)
1600 SEK
Ultrathin sectioning and counter staining for TEM
900 SEK per hour
Full sample preparation for SEM, including drying and coating (max 6 samples)
1500 SEK
SEM sample preparation including only mounting and sputter coating (max 6 samples)
800 SEK
Confocal laser scanning microscopy Leica SP8
1200 SEK per hour
Light sheet microscopy Leica DLS SP8
1200 SEK per hour
Micro-computed tomography (Micro CT) Nikon XTH 225
2500 SEK per hour
Scanning electron microscopy Hitachi SU3500
1400 SEK per hour
Transmission electron microscopy JEOL 1400 Plus
1500 SEK per hour
Staff support during imaging or sample preparation
1400 SEK per hour
Sample preparation and lab support
Fixation and embedding in epoxy for TEM (max 10 samples)
2400 SEK
Ultrathin sectioning and counter staining for TEM
1400 SEK per hour
Full sample preparation for SEM, including drying and coating (max 6 samples)
2200 SEK
SEM sample preparation including only mounting and sputter coating (max 6 samples)
1200 SEK
Our microscope equipment
At our facility we have the following microscopes available for you to use:
- Confocal microscopy and light sheet microscopy for fluorescence imaging at high resolution.
- IR-microscopy for chemical sensitivity measurements of various biological and environmental samples with spatial resolutions ranging from circa 250 μm down to 500 nm.
- X-ray micro-computed tomography (micro-CT), for non-destructive imaging of both large (several decimeters) and small (millimetres) objects, with a resolution below 2 microns for the smallest samples.
- Scanning electron microscopy (SEM) for surface imaging on the nanoscale.
- Transmission electron microscopy (TEM) for high-resolution sub-cellular imaging
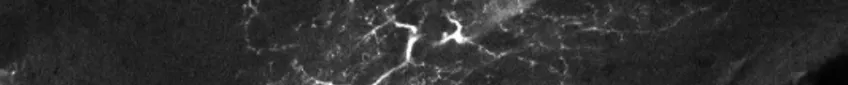
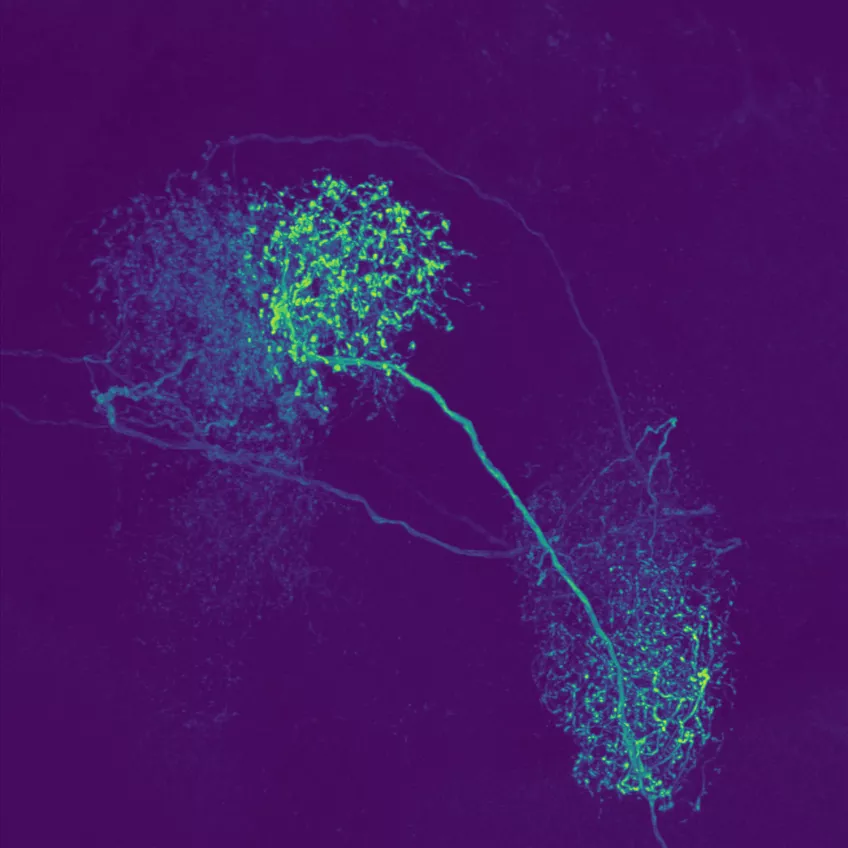
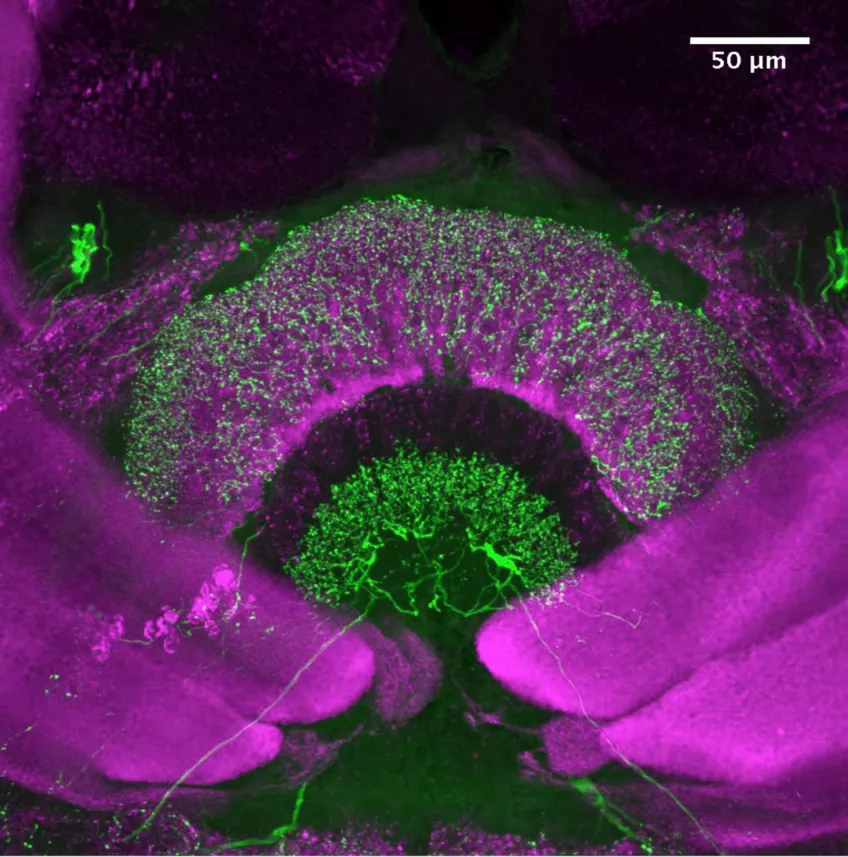
Confocal laser scanning microscopy (CLSM)
Possible application of a confocal laser scanning microscope
Confocal laser scanning microscopy (CLSM) is a method for examining fluorescent or reflecting specimens with clarity. This technique is commonly referred to as confocal microscopy. With this method, you remove out-of-focus light to create sharp, well-defined images of structures within the focal plane, even for specimens that are too thick for traditional fluorescence microscopy.
By capturing a sequence of confocal images at various depths within a thick sample, you can use the power of 3D analysis and rendering. This allows you to see structures that you cannot see through conventional light microscopy.
Usage of the light-sheet function of the confocal laser scanning microscope
Our confocal microscope is also equipped with a light-sheet module. Light sheet microscopy – also referred to as single plane illumination microscopy (SPIM) is a gentle way of imaging sensitive samples or fast biological processes in vivo. The specimen is illuminated only in a single plane at a time and detected from the perpendicular direction. Since there is no out-of-focus excitation, phototoxic effects are reduced to the focal plane. Light sheet imaging has intrinsic optical sectioning. By moving the sample through the light sheet, 3D images of a specimen can be recorded. With this technique, large 3D volumes are captured at a much higher speed but slightly lower resolution compared to confocal imaging.
Details about our confocal laser scanning microscope
Our Leica SP8 DLS (inverted microscope) is equipped with:
- Light source: five lasers with wavelengths:
- 405 nm
- Solid state 488 nm
- Solid state 514 nm
- Solid state 552 nm
- Solid state 638 nm
- Detectors: two PMT:s (photomultiplier tubes), one HyD GaAsP-spectral detector, one PMT for transmitted light
- Objectives for confocal imaging: 10x/0.3, 20x/0.75 IMM, 25x/0.95W, 63x/1.4 Oil, 63x/1.3 GLYC
- Objectives and mirrors for light-sheet microscopy: imaging 25x/0.95W DLS, 10x/0.3W DLS, illumination 2,5x/0.07, 5x/0.15, DLS TwinFlect 2.5mm, DLS TwinFlect 5mm
- Software: LAS X with HyVolution, Dye Finder, 3D Visualisation, Microlab (for FRAP, FLIP and FRET) and Huygens Base Package for Deconvolution
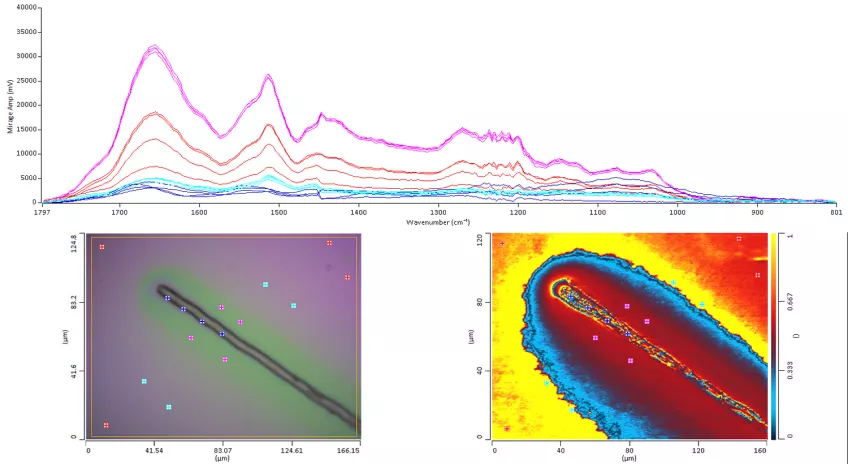
IR-spectroscopy
Infrared Spectroscopy is an analytical tool that examines the interaction between infrared light and molecules, allowing researchers to gain insights through absorption, emission, and reflection analyses. In biology, Infrared Spectroscopy aids in examining biomolecules' specific molecular vibrations, enabling the investigation of various biological samples such as cells, tissues, organs, and biofluids.
Infrared Spectroscopy's label-free and non-invasive nature provides structure-specific spectra for intact cells and tissues. This approach can be used in both qualitative and quantitative analyses. Thus, providing researchers with a powerful means of detecting, classifying, and understanding complex biological systems.
IR spectroscopy measures absolute frequencies at which a sample absorbs radiation, unlike Raman spectroscopy which measures relative frequencies at which a sample scatters radiation.
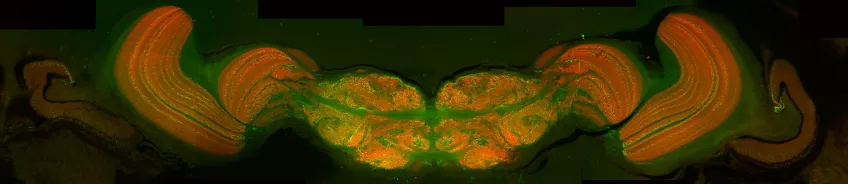
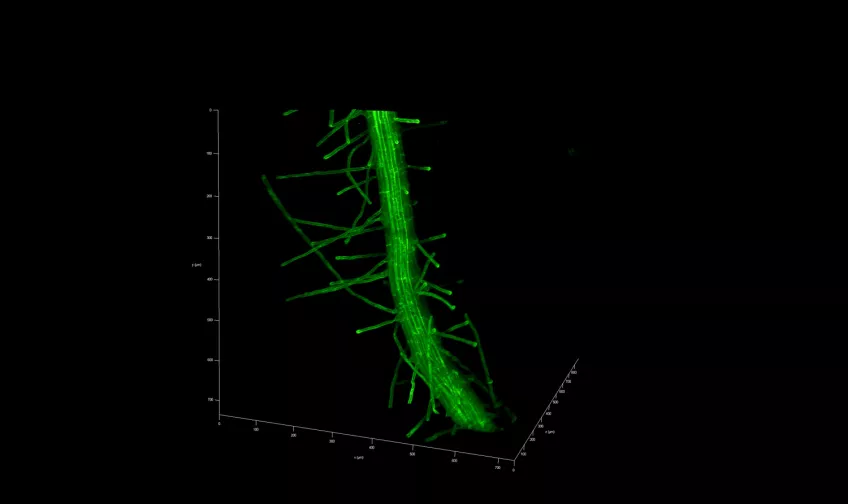
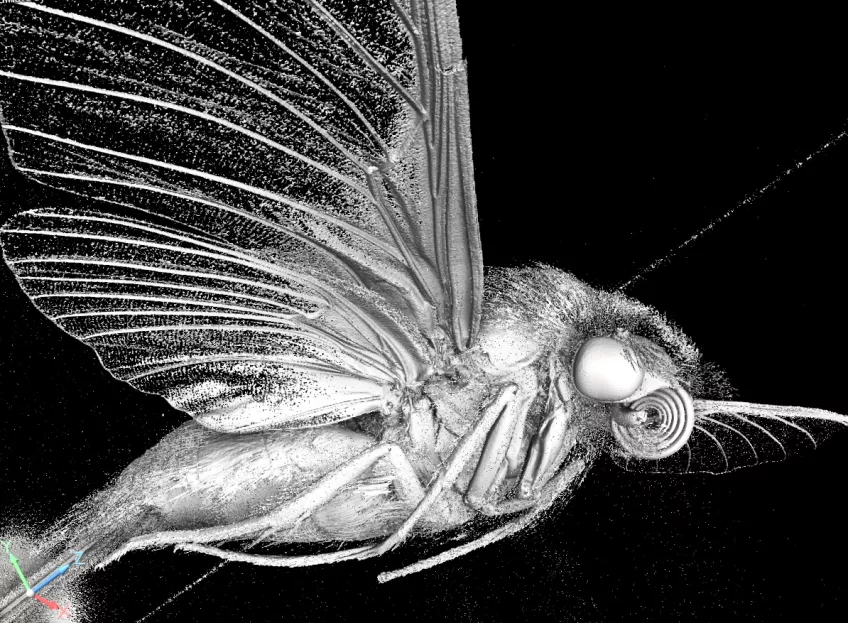
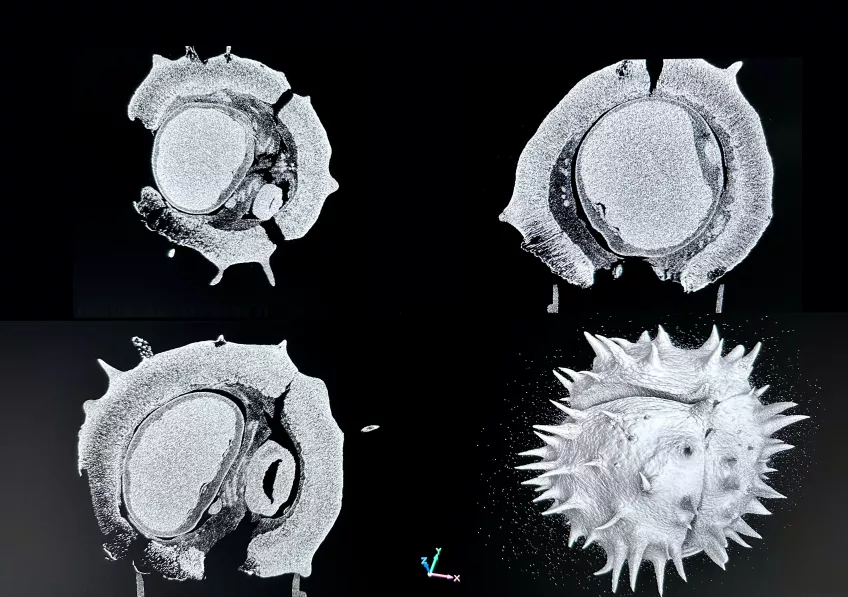
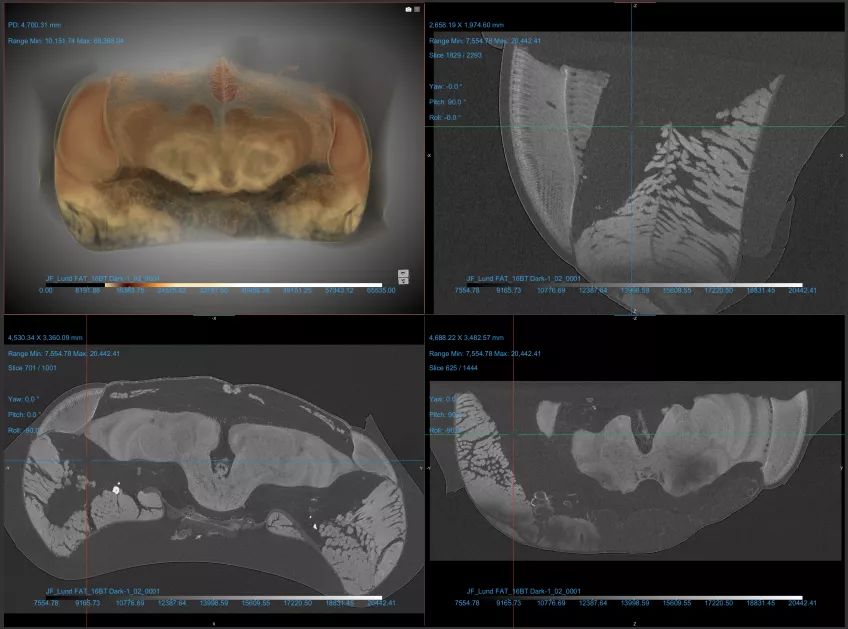
Micro-computed tomography (micro-CT)
Possible application of a micro-computed tomography microscope
Micro-computed tomography (Micro CT) is a non-invasive imaging method used in scientific and industrial fields for detailed 3D visualisation of an object's internal structure. In biology, Micro CT serves as a valuable tool for examining biological specimens without the need for dissection. It enables researchers to explore the inner workings of small organisms, fossils, and biological tissues, contributing to the fields of anatomy, palaeontology, and evolutionary biology. You can also use Micro CT to look at geological and material samples like parts of machines and computers,
Furthermore, in plant biology, Micro CT can help analyse root structures and seed development, providing microscopic insights into plant growth and development. Micro CT plays a role in enhancing our understanding of the natural world by allowing scientists to delve into the hidden details of organisms and fossils.
Details about our micro-computed tomography microscope
With our micro-computed tomography (Micro CT) equipment, Nikon XT H 225, you can x-ray samples in three dimensions. With this non-destructive technique, you can collect and visualise both internal information and external surface information in 3D.
The system is equipped with both a transmission target and a reflection target.
The tungsten transmission target is used for smaller samples (1 cm and below) and higher resolution and can operate at a maximum of 180kV to achieve voxel sizes down to 2 microns.
The reflection target is used for bigger samples (up to approximately 25 cm) and can operate at 225 kV and achieve voxels from 3-125 microns, depending on sample size. For this target, there is a choice of target material (tungsten, molybdenum, silver, and copper) to achieve different X-ray spectra.
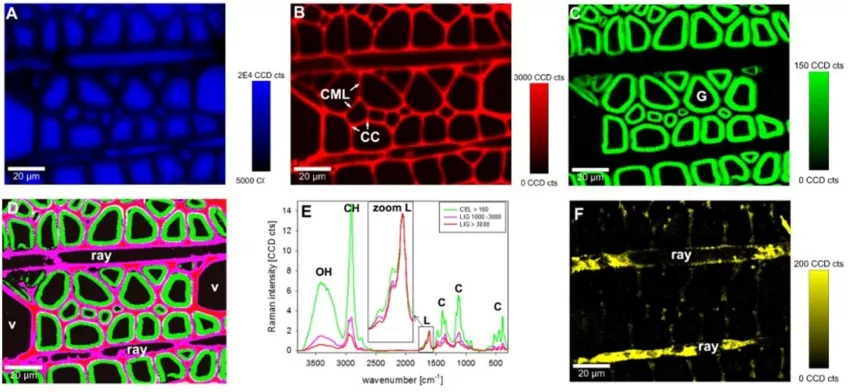
Raman spectroscopy
Raman spectroscopy utilises scattered light to measure the vibrational energy modes of a sample. Thus, providing unique chemical and structural information for substance identification.
In biology, researchers can use this non-destructive technique to identify and distinguish materials. Raman spectroscopy has a spatial resolution of 0.5-1 µm. With Raman spectroscopy, you can examine the structure, function, and dynamics of biomolecules such as proteins and nucleic acids. The technique is non-invasive and has a high chemical specificity.
Raman spectroscopy measures relative frequencies at which a sample scatters radiation, unlike IR spectroscopy which measures absolute frequencies at which a sample absorbs radiation.
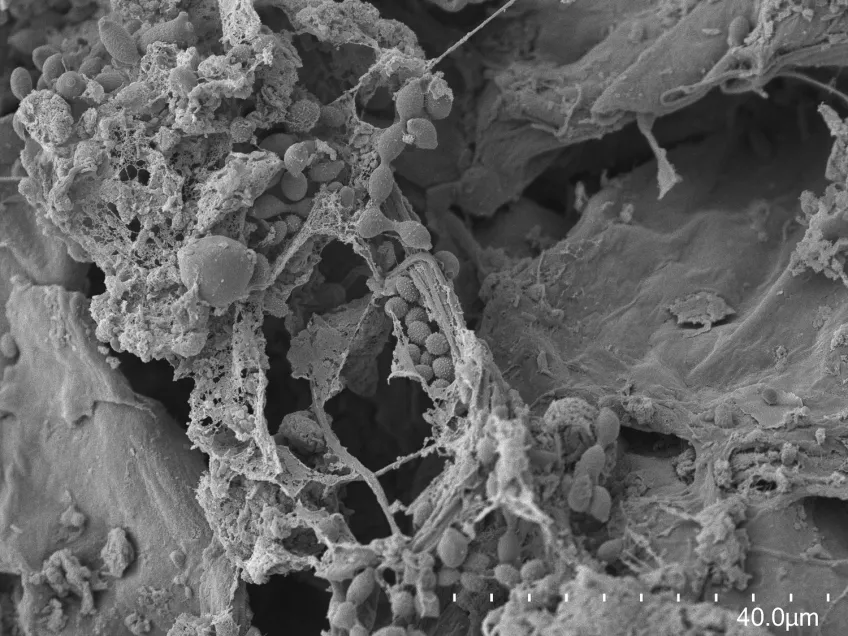
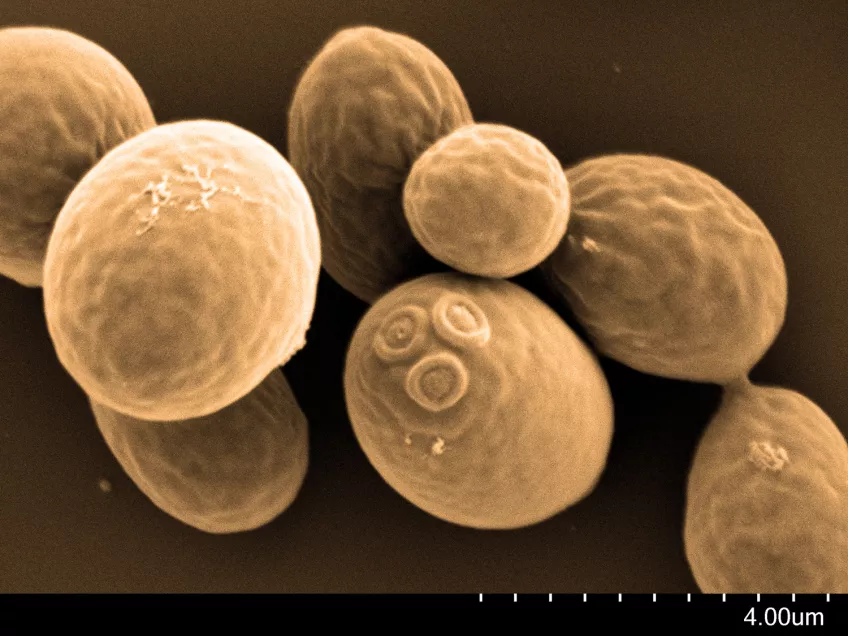
Scanning electron microscopy (SEM)
Scanning electron microscopes (SEMs) are powerful tools used across various scientific and industrial fields. Their main role is to create detailed 3D images of solid specimen surfaces. Engineers and scientists use SEMs to examine the texture and shape of materials. Thus giving us vital information about their structure.
SEMs help biologists explore the surface of cells to study cell structure and surface textures. You can also use SEMs to study interactions between cells and their surroundings. Moreover, SEMs assist in studying tiny structures like hair, pollen, and microorganisms, deepening our understanding of the microscopic world in biology.
Details about our scanning electron microscope
With our Scanning Electron Microscope (SEM), Hitachi SU3500, it is possible to perform both high and low vacuum studies. We can study all types of exposed surfaces of dry specimens like metal-coated samples in high vacuum, and uncoated samples in low vacuum. Typical samples we look at are bacteria, pollen, tissue preparations, insects, and different materials. A resolution of a few nanometers is possible to achieve but is highly dependent on the sample. Operating at low magnifications offers an overview and great depth of focus.
Sample preparation
To dry delicate biological samples with minimal artifacts we have a Leica CPD 300 critical point dryer. To make non-conductive samples conductive we use a Cresstington 208 auto sputter coater equipped with a gold target, creating a few nanometers thin gold layer on top of the samples.
Transmission electron microscopy (TEM)
Scientists use transmission electron microscopes (TEMs) across many scientific fields for ultra-detailed imaging and analysis at the nano-level. We can use TEMs to investigate the internal structure of materials in detail.
At our facility, we commonly use TEMs to look at ultra-thin slices of cells and tissues, organelles, and even viruses at the subcellular level. Moreover, TEMs can also help us characterise nanoparticles and nanomaterials.
Details about our transmission electron microscope
Our Transmission Electron Microscope (TEM) is a 120 kV JEOL JEM 1400 plus, with a bottom-mounted CMOS camera, and both single and penta sample holders. With our TEM we usually study thin sections (50-70 nm) of biological tissue or particle and material specimens like bacteria, viruses, and powder. With the JEOL JEM 1400 plus you can also study sub-nanometer resolutions if the sample allows it.
Sample preparation for TEM
In our lab, we will give support during the entire sample preparation process and provide you with the material, chemicals and equipment needed. We have a Leica UC7 ultramicrotome equipped with a diamond knife for sample sectioning if needed.

Lab manager
Ola Gustafsson
PhD, research engineer
Telephone: +46 46 222 93 43
Email: Ola [dot] Gustafsson [at] biol [dot] lu [dot] se
Acknowledgement
If you are publishing an article with results obtained with the microscopes belonging to the Department of Biology, please make sure to acknowledge us:
"We acknowledge the Microscopy Facility at the Department of Biology, Lund University".